Copper has two isotopes, 63 Cu and 65 Cu. If the atomic mass of copper is 63.5 g/mol, what is the% of each isotope? 25.0% 63 Cu and 75.0% 65 Cu. Copper (Cu) Atomic Data for Copper (Cu) Atomic Number = 29 Atomic Weight = 63.546 Reference E95. Part A 65Cu (atomic Mass = 64.92779 U). Part B 86Sr (atomic Mass = 85.90926 U). Express The Energy In Millions Of Electron Volts To Three Significant Figures For Both Parts. This problem has been solved! The chemical symbol for Copper is Cu. Atomic Mass of Copper. Atomic mass of Copper is 63.546 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
The elements of the periodic table sorted by atomic mass

click on any element's name for further information on chemical properties, environmental data or health effects.
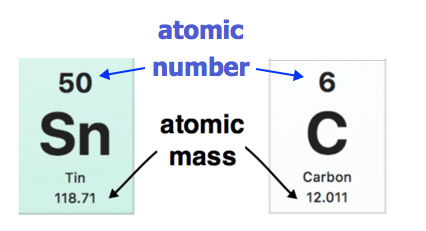
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic Mass | Name chemical element | Symbol | Atomic number |
| - Name alphabetically | 1.0079 | Hydrogen | H | 1 |
| - Atomic number | 4.0026 | Helium | He | 2 |
| - Symbol | 6.941 | Lithium | Li | 3 |
| - Atomic Mass | 9.0122 | Beryllium | Be | 4 |
| - Electronegativity | 10.811 | Boron | B | 5 |
| - Density | 12.0107 | Carbon | C | 6 |
| - Melting point | 14.0067 | Nitrogen | N | 7 |
| - Boiling point | 15.9994 | Oxygen | O | 8 |
| - Vanderwaals radius | 18.9984 | Fluorine | F | 9 |
| - Year of discovery | 20.1797 | Neon | Ne | 10 |
| - Inventor surname | 22.9897 | Sodium | Na | 11 |
| - Elements in earthcrust | 24.305 | Magnesium | Mg | 12 |
| - Elements in human body | 26.9815 | Aluminum | Al | 13 |
| - Covalenz radius | 28.0855 | Silicon | Si | 14 |
| - Ionization energy | 30.9738 | Phosphorus | P | 15 |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). The lightest chemical element is Hydrogen and the heaviest is Hassium. The unity for atomic mass is gram per mol. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 32.065 | Sulfur | S | 16 |
| 35.453 | Chlorine | Cl | 17 | |
| 39.0983 | Potassium | K | 19 | |
| 39.948 | Argon | Ar | 18 | |
| 40.078 | Calcium | Ca | 20 | |
| 44.9559 | Scandium | Sc | 21 | |
| 47.867 | Titanium | Ti | 22 | |
| 50.9415 | Vanadium | V | 23 | |
| 51.9961 | Chromium | Cr | 24 | |
| 54.938 | Manganese | Mn | 25 | |
| 55.845 | Iron | Fe | 26 | |
| 58.6934 | Nickel | Ni | 28 | |
| 58.9332 | Cobalt | Co | 27 | |
| 63.546 | Copper | Cu | 29 | |
| 65.39 | Zinc | Zn | 30 | |
| 69.723 | Gallium | Ga | 31 | |
| 72.64 | Germanium | Ge | 32 | |
| 74.9216 | Arsenic | As | 33 | |
| 78.96 | Selenium | Se | 34 | |
| 79.904 | Bromine | Br | 35 | |
| 83.8 | Krypton | Kr | 36 | |
| 85.4678 | Rubidium | Rb | 37 | |
| 87.62 | Strontium | Sr | 38 | |
| 88.9059 | Yttrium | Y | 39 | |
| 91.224 | Zirconium | Zr | 40 | |
| 92.9064 | Niobium | Nb | 41 | |
| 95.94 | Molybdenum | Mo | 42 | |
| 98 | Technetium | Tc | 43 | |
| 101.07 | Ruthenium | Ru | 44 | |
| 102.9055 | Rhodium | Rh | 45 | |
| 106.42 | Palladium | Pd | 46 | |
| 107.8682 | Silver | Ag | 47 | |
| 112.411 | Cadmium | Cd | 48 | |
| 114.818 | Indium | In | 49 | |
| 118.71 | Tin | Sn | 50 | |
| 121.76 | Antimony | Sb | 51 | |
| 126.9045 | Iodine | I | 53 | |
| 127.6 | Tellurium | Te | 52 | |
| 131.293 | Xenon | Xe | 54 | |
| 132.9055 | Cesium | Cs | 55 | |
| 137.327 | Barium | Ba | 56 | |
| 138.9055 | Lanthanum | La | 57 | |
| 140.116 | Cerium | Ce | 58 | |
| 140.9077 | Praseodymium | Pr | 59 | |
| 144.24 | Neodymium | Nd | 60 | |
| 145 | Promethium | Pm | 61 | |
| 150.36 | Samarium | Sm | 62 | |
| 151.964 | Europium | Eu | 63 | |
| 157.25 | Gadolinium | Gd | 64 | |
| 158.9253 | Terbium | Tb | 65 | |
| 162.5 | Dysprosium | Dy | 66 | |
| 164.9303 | Holmium | Ho | 67 | |
| 167.259 | Erbium | Er | 68 | |
| 168.9342 | Thulium | Tm | 69 | |
| 173.04 | Ytterbium | Yb | 70 | |
| 174.967 | Lutetium | Lu | 71 | |
| 178.49 | Hafnium | Hf | 72 | |
| 180.9479 | Tantalum | Ta | 73 | |
| 183.84 | Tungsten | W | 74 | |
| 186.207 | Rhenium | Re | 75 | |
| 190.23 | Osmium | Os | 76 | |
| 192.217 | Iridium | Ir | 77 | |
| 195.078 | Platinum | Pt | 78 | |
| 196.9665 | Gold | Au | 79 | |
| 200.59 | Mercury | Hg | 80 | |
| 204.3833 | Thallium | Tl | 81 | |
| 207.2 | Lead | Pb | 82 | |
| 208.9804 | Bismuth | Bi | 83 | |
| 209 | Polonium | Po | 84 | |
| 210 | Astatine | At | 85 | |
| 222 | Radon | Rn | 86 | |
| 223 | Francium | Fr | 87 | |
| 226 | Radium | Ra | 88 | |
| 227 | Actinium | Ac | 89 | |
| 231.0359 | Protactinium | Pa | 91 | |
| 232.0381 | Thorium | Th | 90 | |
| 237 | Neptunium | Np | 93 | |
| 238.0289 | Uranium | U | 92 | |
| 243 | Americium | Am | 95 | |
| 244 | Plutonium | Pu | 94 | |
| 247 | Curium | Cm | 96 | |
| 247 | Berkelium | Bk | 97 | |
| 251 | Californium | Cf | 98 | |
| 252 | Einsteinium | Es | 99 | |
| 257 | Fermium | Fm | 100 | |
| 258 | Mendelevium | Md | 101 | |
| 259 | Nobelium | No | 102 | |
| 261 | Rutherfordium | Rf | 104 | |
| 262 | Lawrencium | Lr | 103 | |
| 262 | Dubnium | Db | 105 | |
| 264 | Bohrium | Bh | 107 | |
| 266 | Seaborgium | Sg | 106 | |
| 268 | Meitnerium | Mt | 109 | |
| 272 | Roentgenium | Rg | 111 | |
| 277 | Hassium | Hs | 108 | |
| Darmstadtium | Ds | 110 | ||
| Copernicium | Cn | 112 | ||
| Nihonium | Nh | 113 | ||
| Flerovium | Fl | 114 | ||
| Moscovium | Mc | 115 | ||
| Livermorium | Lv | 116 | ||
| Tennessine | Ts | 117 | ||
| Oganesson | Og | 118 |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
What Is The Atomic Mass Of Copper
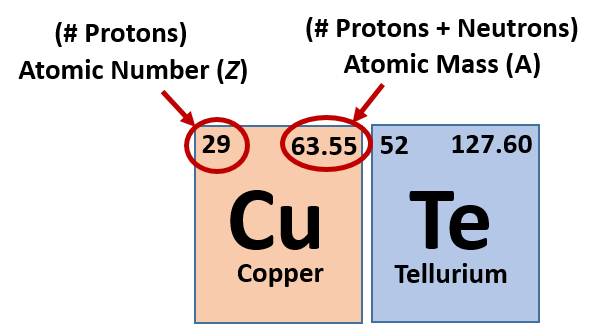
Cu Element Atomic Mass
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copper Atomic Mass 64
Copyright © 1998-2021 Lenntech B.V. All rights reserved
